Extraembryonic Epithelia: a model to study the genetic and developmental basis of morphogenic evolution
The extraembryonic epithelia of flies (serosa and amnion) are an excellent model to study the genetic and developmental basis of change in animal morphogenesis. They also provide a unique opportunity to study how tissue-specific gene regulatory gene networks can merge. We are interested in all aspects of extraembryonic epithelia and study their genetic, morphogenetic, and functional properties in different fly species to understand how genetic and cellular mechanisms of development enabled their evolutionary change. Three types of extraembryonic tissue organizations have been found in flies (Diptera). Two major reorganizations can account for this diversity, one in the stem lineage of the Cyclorrhapha and one in the stem lineage of the Schizophora, as shown in the accompanying figure. The extraembryonic tissue of Drosophila melanogaster is called amnioserosa. This epithelium resulted from merging features of serosa and amnion development, and offers an excellent model for studying the intricate ways in which tissue specific gene regulatory networks can merge in the course of evolution.
Bone Morphogenetic Proteins (BMPs) pattern the dorsal-ventral axis of bilaterian embryos; however, their roles in the evolution of body plan are largely unknown. We examine how the BMP gradients of fly embryos evolve. In fly embryos, BMP signaling specifies extraembryonic tissues. In basal-branching flies such as Megaselia abdita, BMP specifies two tissues: the serosa and amnion. However, in Drosophila melanogaster, BMP signaling specifies a single tissue called the amnioserosa. The BMP signaling dynamics are similar in both species until the beginning of gastrulation when BMP signaling broadens and intensifies at the edge of the germ rudiment in Megaselia while remaining static in Drosophila.
We found that the differences in gradient dynamics and tissue specification result from evolutionary changes in the gene regulatory network that controls the activity of a positive feedback circuit on BMP signaling, involving the tumor necrosis factor alpha homolog eiger. These data illustrate an evolutionary mechanism by which spatiotemporal changes in morphogen gradients can guide tissue complexity. The principle of driving evolution through changes in positive feedback circuits of morphogen gradients might apply in many species but requires a combination of comparative, quantitative, and functional studies for which fly embryos have unique advantages.
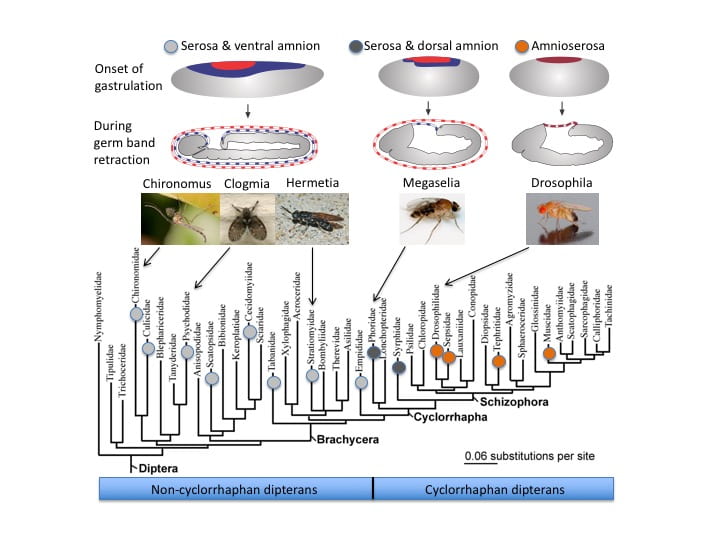
Figure: Phylogenetic occurrence of three extraembryonic tissue topographies in flies (Diptera).
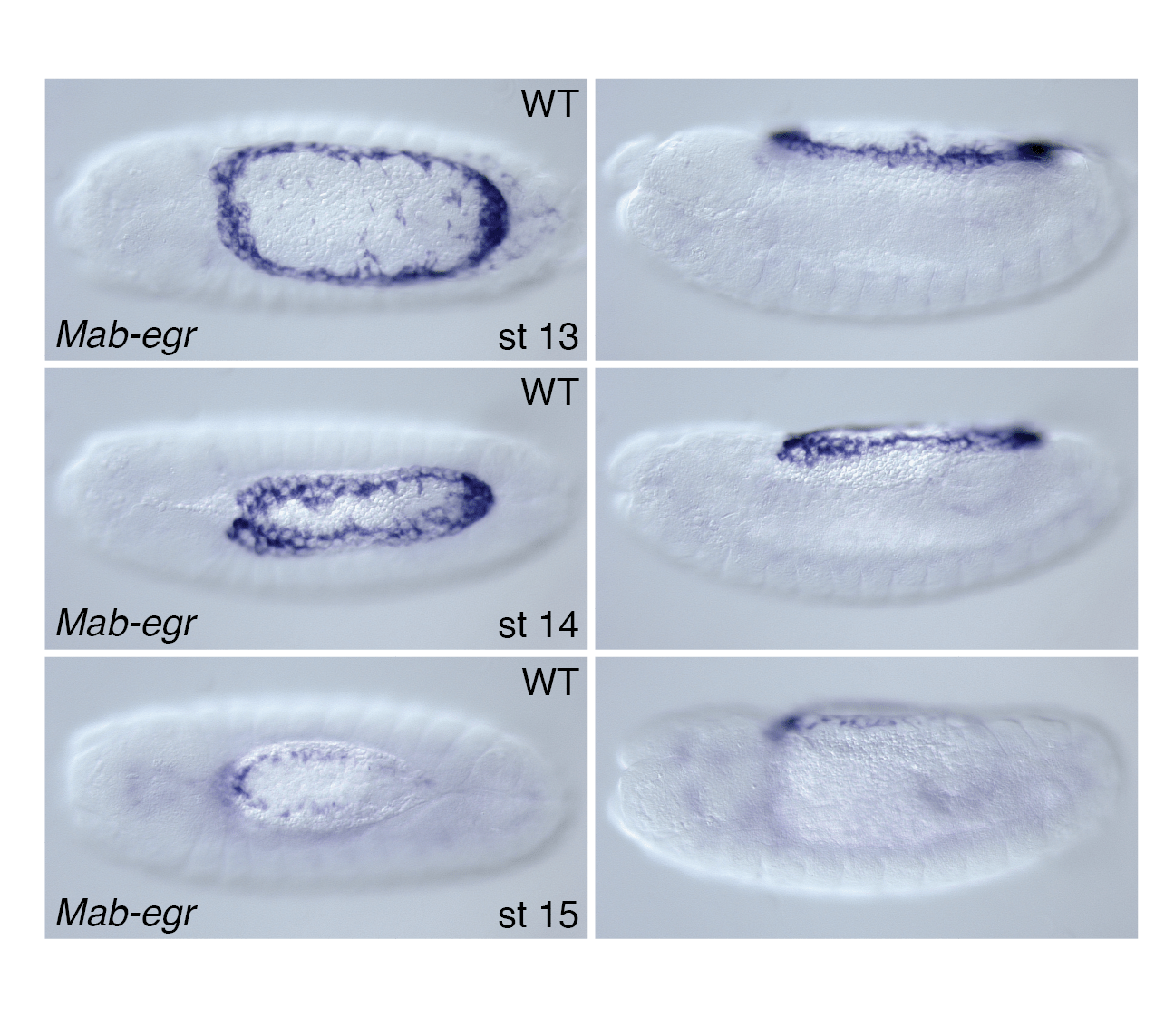
Figure: Transcription of eiger in the amnion of a cyclorrhaphan fly, Megaselia abdita (Phoridae). Dorsal views with anterior to the left.
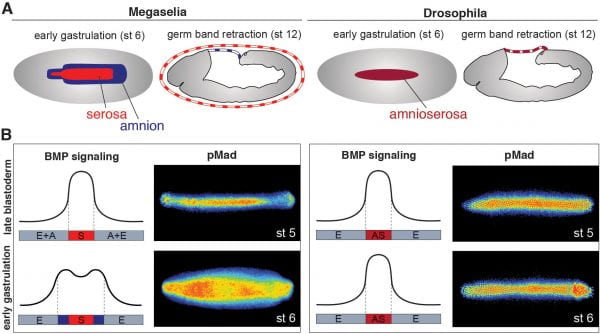
Figure: BMP signaling
Publications
- How two extraembryonic epithelia became one: serosa and amnion features and functions of Drosophila‘s amnioserosa. Philos Trans R Soc Lond B Biol Sci. 2022 Dec 5;377(1865):20210265. doi: 10.1098/rstb.2021.0265. Epub 2022 Oct 17. PMID: 36252222
- Functional evolution of a morphogenetic gradient. Elife. 2016 Dec 22;5:e20894. doi: 10.7554/eLife.20894. PMID: 28005004
- Morphogenetic functions of extraembryonic membranes in insects. Schmidt-Ott U, Kwan CW. Current Opinion in Insect Science. 2016; 13:86-92.
PMID:27436557 - Evolutionary origin of the amnioserosa in cyclorrhaphan flies correlates with spatial and temporal expression changes of zen. Rafiqi AM, Lemke S, Ferguson S, Stauber M, Schmidt-Ott U. Proceedings of the National Academy of Sciences of the United States of America. 2008; 105(1):234-9.
PMID:18172205, PMCID:PMC2224192 - Postgastrular zen expression is required to develop distinct amniotic and serosal epithelia in the scuttle fly Megaselia. Rafiqi AM, Lemke S, Schmidt-Ott U. Developmental Biology. 2010; 341(1):282-90.
PMID:20144604 - The generation of variation and the developmental basis for evolutionary novelty. Hallgrímsson B, Jamniczky HA, Young NM, Rolian C, Schmidt-Ott U, Marcucio RS. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution. 2012; 318(6):501-17.
PMID:22649039, PMCID:PMC3648206 - BMP-dependent serosa and amnion specification in the scuttle fly Megaselia abdita. Rafiqi AM, Park CH, Kwan CW, Lemke S, Schmidt-Ott U. Development. 2012; 139(18):3373-82.
PMID:22874914
